
If methane is modified so that the central carbon is attached to four different groups, the molecule can exist as 2 different isomers that are non-superimposable mirror images (this is called “optical isomerism” and covered later in the course). Disproving The Square Planar Structure Of CH 4 (1874) And Proposal Of A Tetrahedral Structure Extremely brilliant chemists such as Berzelius went to their graves having no reason to doubt that methane was anything but flat. This was in fact the majority opinion for the arrangement of bonds around carbon until about 1880. (We call this structure “square planar”). If all C-H bonds are of equal lengths and angles, why can’t CH 4 have the structure below, where all the bond angles are 90° and CH 4 is flat, in the plane of the page. Maybe Methane (CH 4) Is Square Planar?Īlright, you say. This tells us that all the bond lengths and bond angles in methane are identical. Therefore this cannot be the correct structure. However, the measured dipole moment of CH 4 is zero.

If the above structure accurately depicted the structure of methane, we’d expect methane to have 3 longer C–H bonds (to the 2p orbitals) and one shorter C–H bond (to the 2s orbital, which is closer to the nucleus).We expect C to be partially negative and H to be partially positive. Recall that each C–H bond has a small dipole due to the difference in electronegativity between C (2.5) and H (2.2). Shouldn’t we expect that the structure of methane would have three C-H bonds for each of the p orbitals (at 90 degrees to each other) and then the fourth C-H bond attached to the 2s orbital? Since electron pairs repel, maybe we should put that C-H bond the maximum distance away from the other C-H bonds this would give an H–C–H bond angle of 135°.įollowing this logic would give a structure like this:Īs it turns out, it can be shown that this proposal is wrong. The 3 p-orbitals in carbon are all at 90 degrees to each other, along the x, y, and z axes. So let’s bring four hydrogen atoms into the picture and try to apply what we’ve learned to come up with some hypotheses about the bonding in this molecule. The simplest organic compound is methane, CH 4. This is fine if we’re just talking about isolated carbon atoms.īut in order to be truly useful, we need to be able to relate the orbitals of carbon to the structure and bonding of actual organic compounds. Can We Use This Information To Figure Out The Structure Of Methane (CH 4)? (Spoiler: No) That means that there are two electrons in the 2s orbital, and a single electron in two of the three 2p orbitals. Since the 2s orbital is lower in energy than 2p, it’s filled first. In our review of atomic orbitals, we saw that the orbital configuration of the valence electrons of carbon is 2s 22p 2 as shown below: The Electronic Configuration Of The Valence Electrons Of Carbon Is 2s 22p 2 Tetrahedral Carbons: Not A Popular Idea In 1874ġ.Disproving The Square Planar Structure Of CH 4 (1874) And Proposal Of A Tetrahedral Structure.Can We Use This Information To Figure Out The Structure Of Methane (CH 4)? (Spoiler: No).The Electronic Configuration Of The Valence Electrons Of Carbon Is 2s 22p 2.First, how do we know that CH 4 is tetrahedral? And secondly, how do we reconcile this electronic configuration (2s 22p 2 ) with the fact that we have four equal C–H bonds? It turns out that methane is tetrahedral, with 4 equal bond angles of 109.5° and 4 equal bond lengths, and no dipole moment. If the orbital configuration of carbon is 2s 22p 2 , then how can we use this information to figure out what the arrangement of the orbitals are in a simple organic molecule like methane (CH 4)? (Hint: since the dipole moment of CH 4 is zero, the answer is, “not enough”)
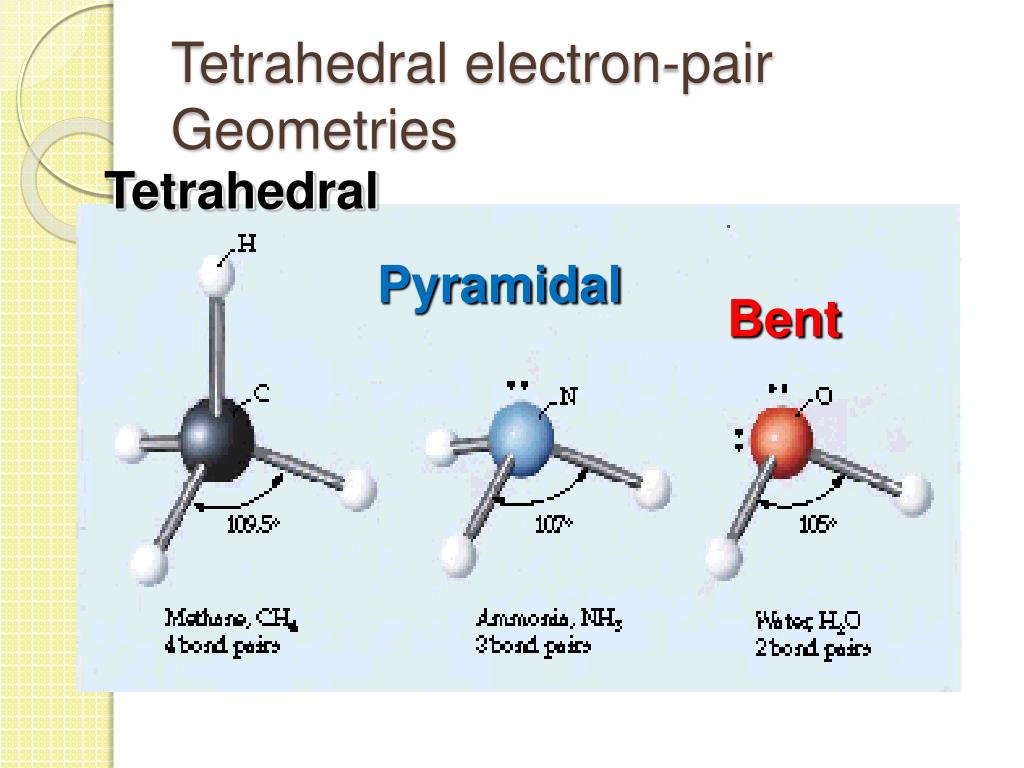
What Do The Valence Electrons Of Carbon Tell Us About The Bonding In CH 4?


 0 kommentar(er)
0 kommentar(er)
